The costliness of drug development and the limitations of studying physiological processes in the lab are two separate scientific issues that may share the same solution.
Microphysical systems (MPSs) are in vitro platforms made up of cells in a microenvironment that closely mimics that found in the body, allowing scientists to recreate the conditions of tissues found within the body for both further elucidation of biological conditions and systems and for applications such as testing drugs in a more precise model than animal testing allows. However, the advancements that MPSs could provide have been limited up to this point by an inability to accurately record exactly what is happening at a cellular level. Now, a team of scientists has developed an electrochemical sensing platform that could solve this issue.
The results were published in Biosensors and Bioelectronics on October 29, 2022.
"Recent bioengineering techniques have realized a construction of tissue model integrated with a perfusable vascular network," said corresponding author Yuji Nashimoto, formally of the Frontier 雷速体育_中国足彩网¥在线直播 Institute for Interdisciplinary Sciences at Tohoku University, now at Tokyo Medical and Dental University. "However, to utilize the models as drug screening tools, we need biosensors to monitor their functions in real-time, which until now were lacking. This study developed new electrochemical sensing platform to monitor the vascularized tissue model."
The team identified electrochemical sensors as ideal for cell functionality readouts because of their low invasiveness, real-time detection and high sensitivity for in vitro culture platforms. Integrating electrochemical sensors into MPSs, however, has been difficult because of their incompatibility with microfluidic devices, according to the researchers.
The researchers were able to integrate their sensing platform for 3D cultured cells with a perfusable vascular network - an engineered vascular system that includes the passage of fluids through it - to measure oxygen metabolism in 3D tissues with vascular flow that mimics that in the human body in real-time.
This successful integration was achieved in part by designing the system to have an open top and a lower layer with five channels for culturing the vascular network and an upper layer that was used for both culturing 3D cultured cells and for oxygen metabolism analysis. The two layers were separated by a thin membrane.
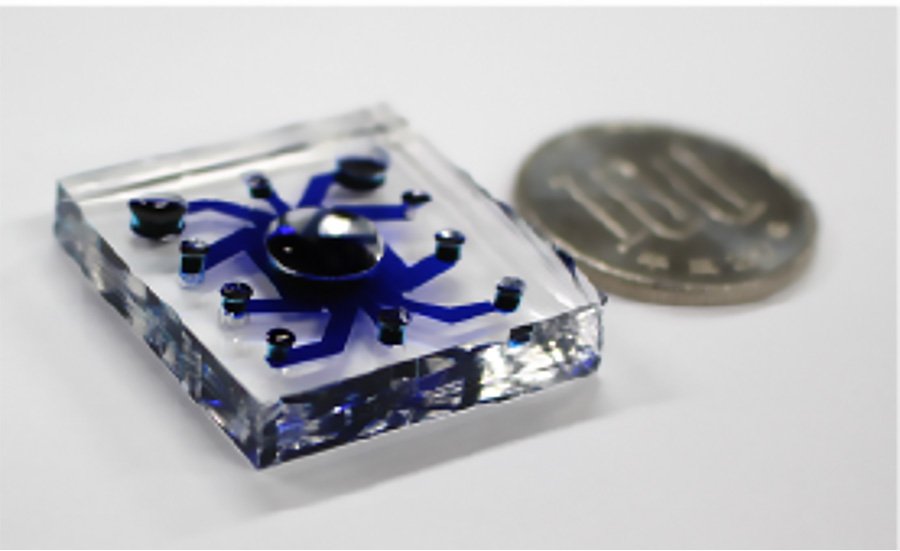
The researchers tested the platform with human lung fibroblast spheroids. They then applied it to a cancer organoid and evaluated the oxygen metabolism changes during drug administration through the vascular network. The results showed that their sensors were successfully integrated into the system to provide the desired accurate measurements.
"We found that the platform could integrate a perfusable vascular network with 3D cultured cells, and the electrochemical sensor could detect the change in oxygen metabolism in a quantitative, non-invasive and real-time manner," said corresponding author Hitoshi Shiku of the Graduate School of Engineering and of the Graduate School of Environmental Studies, both at Tohoku University. "Biosensors are very important tools to realize more physiological drug screening. Our research group has developed various sensors for the purpose. We continue to expand the detectable molecules and to develop more robust and high-throughput sensors."
According to the researchers, future studies should include ways to address the changes of the spheroid and organoid during device culture as well as the development of a perfusable vascular network in an even more controlled environment than currently possible. While the researchers identified the next steps for future studies, the results of this study hold promise for monitoring perfusable vascular networks for drug testing purposes in a way that was not previously achieved.
"This study developed oxygen metabolism analysis for the vascularized tissue model," Shiku said. "In the future, the detectable molecules should be expanded, and the signal-to-noise ratio should be improved."
- Publication Details:
Title: Electrochemical sensing of oxygen metabolism for a three-dimensional cultured model with biomimetic vascular flow
Authors: Yuji Nashimoto, Rei Mukomoto, Takuto Imaizumi, Takato Terai, Shotaro Shishido, Kosuke Ino, Ryuji Yokokawa, Takashi Miura, Kunishige Onuma, Masahiro Inoue, Hitoshi Shiku
Journal: Biosensors and Bioelectronics
DOI: 10.1016/j.bios.2022.114808



 tmd.ac.jp
tmd.ac.jp